Envíos
Lista de comprobación para la preparación de envíos
Como parte del proceso de envío, los autores/as están obligados a comprobar que su envío cumpla todos los elementos que se muestran a continuación. Se devolverán a los autores/as aquellos envíos que no cumplan estas directrices.- Junto al manuscrito (sin autores y sin afiliación institucional) se remiten la página del título y los anexos requeridos por la RMH, según sea el caso (carta de presentación del artículo, declaración jurada, declaración de financiamiento y de conflicto de interés, ICMJE disclosure form, o autorización de publicación del reporte de caso)
- El archivo de envío está en formato de archivo de documento Microsoft Word o similar. El manuscrito está redactado en castellano, portugués o inglés, en formato ISOA4 (212x297 mm), tipo de letra Times New Roman, tamaño de fuente 12 picas, a doble espacio, con márgenes de 25 mm. Páginas numeradas en la parte inferior derecha.
- El manuscrito cumple con la estructura establecida por la RMH; las tablas, los gráficos y las figuras con sus títulos y notas ordenadas secuencialmente se ubican después de las referencias bibliográficas. Las figuras enviadas son de alta resolución.
- Se incluye en el manuscrito una declaración que indique que la investigación fue revisada y aprobada por un organismo de revisión local, regional o nacional independiente; por ejemplo, un comité de ética en investigación o una junta o comité de revisión institucional (Número de Resolución o de documento similar). En los ensayos clínicos se adjunta la constancia de revisión por el Comité de ética en investigación.
- El artículo sin incluir los nombres de los autores y su filiación institucional ha sido evaluado por un programa para detectar similitudes y su índice de similitud es menor de 15%
- Los investigadores indicados como autores han participado en la planificación, investigación, redacción y aprobación del manuscrito, y además, son responsables del contenido de la publicación.
Directrices para autores/as
Revise el tutorial de envío de manuscritos
Consultas: famed.revista.medica@oficinas-upch.pe
INFORMACIÓN E INSTRUCCIONES PARA LOS AUTORES
La Revista Médica Herediana (RMH) es una publicación científica de la Facultad de Medicina Alberto Hurtado de la Universidad Peruana Cayetano Heredia, cuya misión es la difusión de trabajos originales e inéditos que contribuyan al conocimiento de las ciencias biomédicas en especial de la Medicina clínica, de la Salud Pública y de la educación médica, realizados a nivel nacional e internacional. Es arbitrada por pares, de periodicidad trimestral y de acceso abierto. Recibe artículos en español, inglés y portugués.
Antes de iniciar la preparación del manuscrito leer cuidadosamente la Política editorial y las políticas de la ética en investigación y publicación establecidas por la revista y verificar su cumplimiento. Asimismo, revise los aspectos del proceso editorial de la RMH.
Los autores deben indicar si utilizaron tecnologías asistidas por Inteligencia Artificial (IA) como Large Language Model (LLM), chatbots o creadores de imágenes, en la producción del trabajo enviado. Los autores que utilicen dichas tecnologías deben describir, tanto en la carta de presentación como en el trabajo enviado, en la sección correspondiente, y cómo las utilizaron.
La Revista Médica Herediana no acepta la inclusión o el retiro de autores después de haberse iniciado el proceso editorial de los manuscritos. Asimismo, no acepta la modificación de la filiación institucional ni de los grados o títulos.
TIPOS DE MANUSCRITOS
La Revista Médica Herediana publica los siguientes tipos de artículos:
- Editorial (Por encargo)
- Artículo de investigación original: trabajo inédito que describe o muestra resultados de una investigación científica en el área biomédica que brinda información nueva sobre algún tema específico y aporta al conocimiento científico.
- Comunicación corta: informes poco extensos de resultados parciales o finales de una investigación descriptiva de extensión corta, avance u otras observaciones de interés científico.
- Reporte de casos: son presentaciones de casos de uno o varios pacientes con alguna particularidad diagnóstica o terapéutica importante de comunicar, con una breve revisión de publicaciones similares.
- Imágenes en Medicina: es un trabajo que muestra imágenes del examen clínico o de algún examen de diagnóstico por imágenes de importancia para el conocimiento médico. Se acompaña de una breve descripción del caso y de la imagen.
- Reseña histórica: es un manuscrito de algún personaje o hecho ocurrido y su contribución al desarrollo de las ciencias biomédicas o de las políticas en salud.
- Revisión de temas: presenta el estado actual del conocimiento sobre un tema o enfermedad; puede ser de dos tipos:
- solicitada directamente por el Consejo Editorial a personas expertas en el tema,
- presentada por profesionales interesados en un tema en particular. En este caso, el artículo será revisado por especialistas en el tema. Se publican en la sección Contribución especial.
- Conversatorio clínico-patológico (Por editor de sección): se presenta el caso de un paciente y la discusión del diagnóstico diferencial con comentarios de los especialistas que participaron en la evaluación del paciente. Se revisan los hallazgos de los exámenes de laboratorio, de imágenes y de anatomía patológica.
- Cartas al editor: los lectores pueden enviar comentarios sobre algún artículo publicado en la revista o sobre el tema de un artículo publicado o de interés biomédico. La decisión sobre la publicación de las cartas recibidas queda a discreción del Consejo Editorial.
PRESENTACIÓN Y ENVÍO DEL MANUSCRITO
El manuscrito (sin los nombres de los autores, de la institución donde se realizó el estudio, del autor corresponsal y de la contribución de autoría) debe ser enviado junto con los siguientes documentos:
- Página del Título. La página del título se presenta en archivo aparte. Debe contener: Título del artículo, título corto, nombre completo del autor o autores, institución donde se realizó el estudio y la contribución de autoría (según los criterios del International Committee of Medical Journal Editors). Además, información del autor corresponsal, recuento de palabras y número de tablas, gráficos y figuras.
- Carta de presentación del artículo (según formato establecido). Dirigida al Editor jefe de la Revista Médica Herediana, firmada por uno de los autores, solicitando la evaluación para ser considerada su publicación. La carta debe incluir el título del trabajo, el nombre completo de los autores y tipo de articulo. Además, indicar si el manuscrito es parte de o se basa en una tesis de grado o título y si se utilizaron herramientas basadas en inteligencia artificial (IA) en su elaboración.
- Declaracion jurada (según formato establecido). Se declara que el artículo presentado es propiedad intelectual de los autores, que no ha sido publicado, ni presentado para evaluación en otra revista, cediendo los derechos de edición, publicación y difusión a la Revista Médica Herediana en caso el manuscrito sea aceptado para publicación. Asimismo, declaran que cumplen con la política de reconocimiento de autoría de la Revista y se comprometen . a no incluir ni retirar nombres de autores después de haberse iniciado el proceso editorial. Será firmada por todos los autores.
- Declaración de financiamiento y de conflictos de intereses (según formato establecido). Declaran que el estudio fue financiado por los investigadores y que no han recibido financiación o apoyo de cualquier tipo. Si el estudio de investigación recibió financiación o algún tipo de apoyo desde la planificación inicial o durante el desarrollo del mismo, se debe llenar el ICMJE DISCLOSURE FORM. Además, cada autor que recibió algún tipo de apoyo en los últimos 36 meses, debe llenar el ICMJE DISCLOSURE FORM.
- Autorización de publicación de Reporte de caso (según formato). Firmado por el paciente, en el caso de los reportes de casos.
Dichos Formatos se encuentran disponibles para descarga en la parte superior de este sitio web.
Al someter un manuscrito para publicación en la Revista Médica Herediana, los autores aceptan con su firma, explícita o implícitamente, que:
- Conocen las instrucciones para los autores y las han seguido detalladamente.
- Todos los autores cumplen todos los criterios internacionalmente aceptados para ser considerados como tal.
- No se ha excluido de la lista de autores el nombre de alguna persona que reúna los requisitos para ser autor.
- Todos los autores conocen la versión final del manuscrito sometido para publicación y están de acuerdo con ella.
- No se ha incurrido en conducta alguna que pueda considerarse como transgresión de la integridad científica o de los principios éticos que rigen las publicaciones científicas.
- Se comprometen a no incluir ni retirar nombres de autores después de haberse iniciado el proceso editorial.
El envío de manuscritos se realiza a través de la plataforma web de la revista (Open Journal System – OJS) (ver tutorial) disponible en: https://revistas.upch.edu.pe/index.php/RMH/about/submissions
DEL MANUSCRITO
El manuscrito debe pertenecer a una de las siguientes categorías:
- Artículo de investigación original
- Comunicación corta
- Reporte de caso
- Imágenes en Medicina
- Reseña histórica
- Revisión de temas
- Carta al editor
PREPARACIÓN DE LOS MANUSCRITOS
El artículo debe ser redactado en castellano, portugués o inglés, en MS Word o similar, página tamaño A4, tipo de letra Times New Roman, tamaño de fuente 12 picas, a doble espacio, con márgenes de 25 mm y en una sola columna. Cada parte del artículo debe empezar en página aparte.
Las fracciones decimales se deben separar de los números enteros con coma decimal y los miles y millones por un espacio simple. En el texto en inglés las fracciones decimales se separan de los enteros con punto. Las unidades de medida deben ser expresadas mediante el Sistema Internacional de Unidades (SI).
Las tablas, gráficos y figuras con su título correspondiente, se colocan al final del texto en páginas aparte; no deben ser insertados dentro del texto.
Los autores deben seguir las recomendaciones del International Committee of Medical Journal Editors, Preparing a Manuscript for Submission to a Medical Journal.
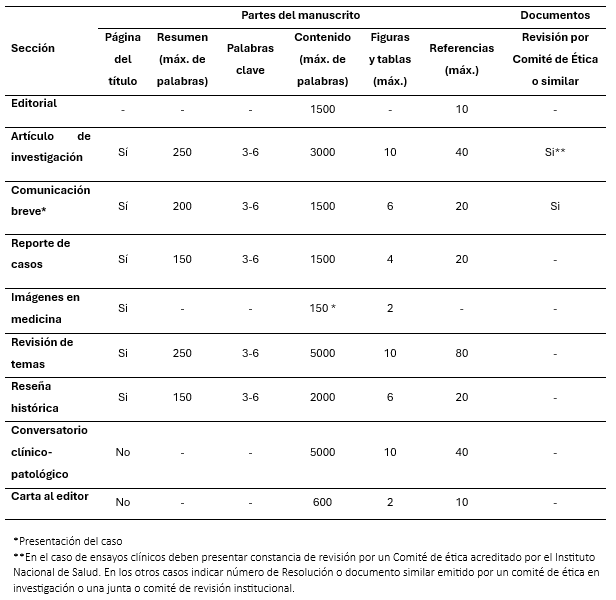
Secciones del manuscrito
Extensión y necesidad de revisión por Comité de ética
Página del Título
La página del título se presenta en archivo aparte. Debe contener: Título del artículo, nombre completo del autor o autores, institución donde se realizó el estudio, y la contribución de autoría. Además, información del autor corresponsal, recuento de palabras y número de tablas, gráficos y figuras.
- Título del artículo en el idioma original e inglés, no mayor de 20 palabras
- Título corto: en el idioma original
- Nombre del autor o autores: seguir el siguiente orden: primer nombre y apellido paterno. Si el autor desea usar su apellido materno puede hacerlo a continuación de su apellido paterno uniéndolo con un guion. Los autores se deben separar por una coma. A continuación del nombre del autor se debe colocar la afiliación institucional (máximo 2 instituciones) usando números, seguido de la letra que indica el título profesional o mayor grado académico obtenido, ambos en superíndice y separados por una coma.
- Nombre de la institución o instituciones, a las que tiene afiliación el autor precedido por el número del llamado correspondiente en superíndice;
- Grado más alto alcanzado o Título profesional del autor precedido por la letra del llamado correspondiente en superíndice.
Ejemplo: Juana Pérez-Sosa 1, a
1 Facultad de Medicina, Universidad Nacional Mayor de San Marcos. Lima, Perú.
a Docente
- ORCID ID de cada autor
- Correspondencia: se debe colocar el nombre del autor corresponsal, dirección postal y correo electrónico; si desea, puede colocar el número de teléfono.
- Contribución de autoría: Se deben colocar las iniciales de cada autor seguidas de la contribución a la investigación y redacción del manuscrito, según los criterios establecidos en las recomendaciones para los autores del International Committee of Medical Journal Editors (ICMJE) https://www.icmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of-authors-and-contributors.html.
- Recuento de palabras: número de palabras del texto del artículo, excluyendo su resumen, agradecimientos, tablas, leyendas de figuras y referencias.
- Número de tablas, gráficos y figuras: mencionar el número de figuras y tablas.
a) Resumen
Las investigaciones originales, las revisiones sistemáticas y los metanálisis requieren resúmenes estructurados. El resumen debe proporcionar el contexto o los antecedentes del estudio y debe indicar el objetivo del estudio, los procedimientos básicos (selección de los participantes del estudio, entornos, mediciones, métodos analíticos), los principales hallazgos y principales conclusiones. Debe enfatizar aspectos nuevos e importantes del estudio o de las observaciones, señalar limitaciones importantes y no sobre interpretar los hallazgos. Los resúmenes de ensayos clínicos deben incluir elementos que el grupo CONSORT ha identificado como esenciales.
Los resúmenes estructurados deben tener una extensión máxima de 250 palabras y ser redactados en un solo párrafo e incluir los siguientes subtítulos: Objetivo, material y métodos, resultados y conclusiones.
En los ensayos clínicos se debe publicar el número de registro del ensayo clínico al final del resumen. Si los datos se han depositado en un repositorio público y/o se están utilizando en un análisis secundario, los autores deben indicar al final del resumen el identificador único y persistente del conjunto de datos, el nombre y el número del repositorio.
En el caso de reportes de caso, reseña histórica y revisión de temas, el resumen es no estructurado, debe tener una extensión máxima de 150 palabras y ser redactado en un solo párrafo.
El resumen se redacta en tiempo pasado.
b) Palabras clave
Las palabras clave son términos o frases cortas que permiten clasificar y direccionar las entradas en los sistemas de indexación y de recuperación de la información en las bases de datos de un manuscrito o área temática en particular.
Las palabras clave deben ser descriptores en Ciencias de la Salud (DeCS) y en ingles en MeSH de la National Library of Medicine (NLM). Deben ser palabras o frases que no se hayan incluido en el título.
c) Introducción
Explicar cuál es el problema, por qué se lleva a cabo la investigación y qué se sabe de la materia antes de emprender la investigación. Dar el fundamento racional o la justificación del estudio. Al final, indicar el objetivo del estudio o la pregunta de investigación redactado en tiempo pasado. Citar solo las referencias pertinentes, las más relevantes.
d) Material y métodos
Debe ser lo suficientemente detallada como para que otras personas con acceso a los datos puedan reproducir el estudio. Describir claramente el tipo y diseño de estudio, la población de estudio, la selección de participantes, los criterios de elegibilidad y exclusión y una descripción de la población de origen. Indicar qué tan representativa es la muestra del estudio de la población de interés.
Debe incluir una declaración que indique que la investigación fue revisada y aprobada por un organismo de revisión local, regional o nacional independiente; por ejemplo, un comité de ética o una junta de revisión institucional.
Mencionar los métodos, equipos (indicando el nombre y la dirección del fabricante entre paréntesis) y procedimientos con suficiente detalle para permitir que otros reproduzcan los resultados.
Dar referencias y descripciones breves de métodos que se han publicado pero que no son bien conocidos; describir métodos nuevos o sustancialmente modificados, dar las razones para usarlos y evaluar sus limitaciones. Si se usaron sustancias químicas o medicamentos identificarlos con precisión con sus nombres genéricos, las dosis y las vías de administración. Identificar nombres científicos y nombres de genes apropiados.
Describir los métodos estadísticos con suficiente detalle. Especifique los programas estadísticos y las versiones utilizadas e indique el tipo de licencia.
La sección material y métodos se redacta en tiempo pasado.
e) Resultados
Presentar los datos representativos, digeridos, discriminados; en secuencia lógica, primero los hallazgos principales o más importantes. No repetir los datos de las tablas, gráficos o figuras en el texto. Proporcionar resultados numéricos no sólo como derivadas (por ejemplo, porcentajes) sino también como números absolutos.
No hacer comentarios. Se redacta en tiempo pasado.
f) Tablas y gráficos
Las tablas, gráficos y figuras deben tener un título breve y claro y serán numeradas según el orden que se indica en el texto. En el texto se colocan entre paréntesis, por ejemplo: (tabla 1); (figura 1). Los títulos deben ser escritos en fuente Times New Roman de 12 picas. El título de la tablas se coloca en la parte superior; en los gráficos y figuras el título se coloca en la parte inferior.
Las tablas se deben elaborar en formato Microsoft Excel insertas con su título correspondiente; sólo deben tener tres líneas horizontales: una debajo del título, otra debajo de los encabezamientos de las columnas y la tercera al final de la tabla. No se deben utilizar líneas verticales.
Las tablas, gráficos o figuras no deben tener recuadro. Se colocan al final del manuscrito, después de la sección Referencias Bibliográficas.
Las figuras y fotos deben ser presentadas en formato TIF, JPG o GIF, en alta resolución. Si la imagen no tiene texto incluido, la resolución óptima para los archivos CMYK es de 300 dpi; si incluye texto, la resolución recomendada es de 600 dpi y, si son de blanco y negro, de 1.200 dpi. En las figuras se deben colocar flechas indicando la lesión o imagen que se quiere mostrar.
En las preparaciones de microscopio, se deben mencionar la coloración y el aumento resultado del producto del objetivo por el aumento del ocular; ejemplo, ocular 10X y objetivo 40X, aumento 400X.
Cuando se utilicen cuadros, tablas, gráficos o figuras que ya hayan sido publicados, se requiere la autorización de la editorial que ostenta los derechos de reproducción.
g) Discusión
Dice qué significan los resultados. La introducción debe haber planteado una o más preguntas; asegurar que la discusión conteste lo que la introducción preguntó.
Es útil comenzar la discusión resumiendo brevemente los principales hallazgos y explorar posibles mecanismos o explicaciones para esos hallazgos.
Enfatizar los aspectos nuevos e importantes de su estudio. Discutir la influencia o asociación de variables, como sexo o género, en sus hallazgos, cuando sea apropiado, y las limitaciones de los datos. Al final, se puede incluir las conclusiones y recomendaciones de los autores.
No se debe repetir en detalle datos u otra información proporcionada en otras partes del manuscrito, como en la sección Introducción o Resultados.
En los reportes de caso, mencionar las fortalezas y limitaciones en su enfoque del caso, especificar si el reporte puede modificar la práctica clínica o las guías de práctica clínica o puede sugerir una hipótesis, mencionar conclusiones de ser posible.
h) Agradecimientos
Cuando en esta sección se nombren personas, los autores deben certificar que ellos tienen conocimiento y están de acuerdo de aparecer en los agradecimientos. Esto no es necesario cuando se nombran entidades.
i) Conflicto de intereses y financiación
Los autores deben incluir en el manuscrito, antes de las referencias bibliográficas, un párrafo en el que expresen si existen conflictos de intereses o si no los hay. Además, mencionar la fuente de financiación de la investigación.
La Revista Médica Herediana, sigue las recomendaciones del ICMJE y adopta el formato de declaración de potenciales conflictos de intereses, el cual debe ser diligenciado individualmente por cada uno de los autores del manuscrito y enviado junto con la carta de presentación del artículo. El formulario electrónico está disponible en http://www.icmje.org/conflicts-of-interest/ o se puede descargar desde la pestaña “Formatos”.
j) Referencias bibliográficas
Después de utilizar en cualquier forma material ajeno, se debe colocar la o las citas de las referencias bibliográficas entre paréntesis, y en orden de aparición consecutiva mediante un número arábigo, en superíndice; por ejemplo: (8) o (6-8), sin colocar hiperenlaces.
Al final del documento, se debe colocar la lista de las referencias bibliográficas citadas en el texto del artículo, numeradas en orden ascendente. Las referencias bibliográficas serán redactadas de acuerdo con las recomendaciones del ICMJE (Vancouver). No se permite la mención de comunicaciones personales, documentos inéditos, ni en prensa (se deben mencionar en el texto, entre paréntesis).
Para ver ejemplos de cómo redactar las referencias bibliográficas se puede ingresar a: http://www.nlm.nih.gov/bsd/uniform_requirements.html
Ejemplos:
Artículo publicado en revista:
Apellido del autor seguido de inicial del nombre, separado por una coma; sólo se coloca punto al final del último autor. Título del artículo. Nombre abreviado de la revista. Año de publicación, punto y coma, volumen seguido del número de fascículo entre paréntesis, dos puntos y finalmente el número de páginas.
Cieza J, Uriol C. Letalidad y la mortalidad de Covid 19 en 60 países afectados y su impacto en los aspectos demográficos, económicos y de salud. Rev Med Hered. 2020; 31(4):214-221.
Listar los seis primeros autores seguido de et. al., si los autores son más de seis.
Rose ME, Huerbin MB, Melick J, Marion DW, Palmer AM, Schiding JK, et al. Regulation of interstitial excitatory amino acid concentrations after cortical contusion injury. Brain Res. 2002;935(1-2):40-6.
Opcional: En el caso que la revista tenga volumen con paginación continua se puede prescindir del número de fascículo.
Cieza J, Uriol C. Letalidad y la mortalidad de Covid 19 en 60 países afectados y su impacto en los aspectos demográficos, económicos y de salud. Rev Med Hered. 2020; 31:214-221.
Artículo publicado en revista con doi:
Si el artículo tiene asignado un doi, colocar solo el doi luego de la metadata del artículo:
Cieza J, Uriol C. Letalidad y la mortalidad de Covid 19 en 60 países afectados y su impacto en los aspectos demográficos, económicos y de salud. Rev Med Hered. 2020; 31(4):214-221. Doi: 10.20453/rmh.v31i4.3852
Artículo publicado en revista en internet:
La metadata de los artículos publicados en versión electrónica deben estar acompañadas de la fecha en la cual se tuvo acceso a la misma.
Cieza J, Uriol C. Letalidad y la mortalidad de Covid 19 en 60 países afectados y su impacto en los aspectos demográficos, económicos y de salud. Rev Med Hered [Internet]. 2020 [Citado el 10 de mayo de 2021]; 31:214-221. Disponible en: https://revistas.upch.edu.pe/index.php/RMH/article/view/3852/4347
Libros:
Autor y coautores en igual forma que para los artículos. título del libro. ciudad donde se editó, dos puntos, nombre de la Editorial, punto y coma, año de publicación. y las páginas consultadas.
Aguilar J. Bases de la inmunología clínica. Lima, Perú: Centro Editorial de la Universidad Peruana Cayetano Heredia; 2019. p. 68.
Capítulos de libros, folletos o similares:
Apellido paterno del autor y/o autores seguido de las iniciales de los nombres (se puede citar hasta seis autores) separado por comas; si son más de seis se anotarán los tres primeros y se agregará “et al.”; se debe colocar un punto al final de la inicial del nombre del último autor y a continuación se citará el título del artículo en el idioma de origen terminando en punto seguido y luego la preposición “En” seguida de dos puntos y el título del libro en el idioma de origen, punto seguido, ciudad donde se editó, dos puntos, nombre de la Editorial, punto y coma, año de publicación, punto y las páginas en las que aparece el trabajo.
García-Moncó J, Erro M, García D, Ezpeleta D. Cuadros clínicos neurológicos asociados a la infección por SARS-CoV-2. En: Ezpeleta D, editor. Manual COVID-19 para el neurólogo general. Madrid: Ediciones SEN; 2020. p. 36-46.
Tesis:
Autor en igual forma que para los artículos. Título del trabajo, punto seguido, especificar el grado optado, punto seguido. Ciudad y país donde se sustentó, separados por una coma, dos puntos y el nombre completo de la Universidad de procedencia, punto y coma, el año, punto seguido, luego el número de páginas, seguido de la abreviatura pp.
Rodríguez M. Cistogastrostomía laparoscópica endoluminal para el tratamiento quirúrgico de las colecciones peripancreáticas. Tesis de Doctor en Medicina. Lima, Perú: Universidad Peruana Cayetano Heredia; 2019. 59 pp.
Páginas electrónicas:
Se debe indicar la fecha en la cual se tuvo acceso a la misma y el URL.
Organización Panamericana de la Salud. Brote de enfermedad por el Coronavirus (COVID-19). Washington DC: Organización Panamericana de la Salud; 2020. (Citado el 22 de enero del 2021) Disponible en: el https://www.paho.org/es/temas/coronavirus/brote-enfermedad-por-coronavirus-covid-19
ENVÍO DEL MANUSCRITO
1. El manuscrito, la página del título, la carta de presentación del artículo, la declaración jurada y la declaración de financiamiento y de conflicto de interés se deben enviar a través del sistema en línea disponible en: https://revistas.upch.edu.pe/index.php/RMH/about/submissions
Los modelos de carta de presentación del artículo, declaración jurada y declaración de financiamiento y de conflicto de interés se pueden descargar desde la pestaña “Formatos”.
2. En caso algún autor o autores haya recibido algún tipo de apoyo en los últimos 36 meses, se debe enviar el ICMJE DISCLOSURE FORM firmado por cada autor del manuscrito que recibió apoyo.
Al someter un manuscrito para publicación en la Revista Médica Herediana, los autores aceptan con su firma, explícita o implícitamente, que:
- Conocen las instrucciones para los autores y las han seguido detalladamente.
- Todos los autores cumplen todos los criterios internacionalmente aceptados para ser considerados como tal.
- No se ha excluido de la lista de autores el nombre de alguna persona que reúna los requisitos para ser autor.
- Todos los autores conocen la versión final del manuscrito sometido para publicación y están de acuerdo con ella.
- No se ha incurrido en conducta alguna que pueda considerarse como transgresión de la integridad científica o de los principios éticos que rigen las publicaciones científicas.
- Se comprometen a no incluir ni retirar nombres de autores después de haberse iniciado el proceso editorial.
ESQUEMAS DE PRESENTACIÓN
Investigación Original
Debe contener las siguientes partes:
- Página del Título
- Resumen (en el idioma original: español o portugués) + palabras clave
- Summary (Abstract) + keywords
- Introducción
- Material y métodos
- Resultados
- Discusión
- Referencias bibliográficas
- Tablas, gráficos y figuras
La extensión total del texto del manuscrito, excluyendo el resumen, agradecimientos, tablas, leyendas de figuras y referencias, no debe ser mayor 3000 palabras (10 páginas escritas a doble espacio, sin incluir tablas, gráficos y figuras). Se acepta como máximo de diez tablas, gráficos o figuras; el número máximo de referencias bibliográficas es 40.
El RESUMEN y el SUMMARY (ABSTRACT), se presentarán cada una en hoja aparte y con una extensión máxima de 250 palabras. Deben ser redactadas en un solo párrafo e incluir los siguientes subtítulos: Objetivo, material y métodos, resultados y conclusiones, y al final se debe agregar 3 a 6 palabras clave o keywords, que ayuden a clasificar el artículo. Las palabras clave deben ser descriptores en Ciencias de la Salud (DECS), las que pueden ser consultadas en https://decs.bvsalud.org/es y en inglés deben ser términos MeSH, las que pueden ser consultadas en https://meshb.nlm.nih.gov/search
La sección INTRODUCCIÓN no debe exceder de dos páginas escritas a doble espacio (máximo 600 palabras). El objetivo del estudio se coloca al final de la introducción, en tiempo pasado y en forma clara y concisa.
La sección MATERIAL Y MÉTODOS debe contener tipo y diseño, población de estudio, criterios de selección, procedimientos, manejo de los datos, análisis estadístico, pruebas estadísticas y programa utilizado. Se debe mencionar si el estudio fue revisado y aprobado por algún Comité de Ética acreditado.
La sección RESULTADOS solo debe mostrar los hallazgos encontrados en el estudio. Solo se deben mostrar los datos más relevantes. No se interpretan ni comentan los hallazgos.
La sección DISCUSIÓN no debe exceder de cuatro páginas escritas a doble espacio (no más de 1200 palabras) y en el último párrafo se redactan las conclusiones del estudio.
Las citas bibliográficas se deben colocar mediante números entre paréntesis, en superíndice, y en orden de aparición.
Comunicación corta o breve
Son informes de resultados parciales de un estudio o resultados finales de una investigación descriptiva de extensión corta o de alguna característica particular de una serie de casos pequeña (menor de 10 casos). Tiene las mismas partes de una investigación original: Página del título, resumen (en el idioma original y en inglés), palabras clave, introducción, métodos, resultados, discusión, referencias bibliográficas y tablas, gráficos y figuras.
La extensión total del texto del manuscrito, excluyendo el resumen, agradecimientos, tablas, leyendas de figuras y referencias, no debe ser mayor de 1 500 palabras (6 páginas escritas a doble espacio, sin incluir tablas, gráficos y figuras). Se acepta como máximo de seis tablas, gráficos o figuras; el número máximo de referencias bibliográficas es 20.
Reporte de casos
Presentación de casos clínicos que destacan alguna particularidad llamativa, señalan un hallazgo especial de las mismas o alguna enfermedad poco frecuente, con una revisión breve de la literatura pertinente. Debe contener las siguientes partes: Página del título, resumen (en el idioma original y en inglés), palabras clave, introducción, presentación del caso, discusión, referencias bibliográficas y tablas, gráficos y figuras.
La extensión total del trabajo, excluyendo el resumen, agradecimientos, tablas, leyendas de figuras y referencias, no debe exceder las 1 500 palabras (6 páginas escritas a doble espacio, sin incluir tablas, gráficos y figuras). Se acepta como máximo de seis tablas, gráficos o figuras; el número máximo de referencias bibliográficas es 20.
Los resúmenes (en el idioma original e inglés) son de tipo no estructurado y no tiene subtítulos; debe indicar el aporte del reporte de caso a la literatura médica y cuál es la principal lección, asi como las características más importantes del caso. La extensión máxima es de 150 palabras y escritos en un solo párrafo. Al final se deben agregar 3 a 6 palabras clave que ayuden a clasificar el artículo, no incluir términos que se mencionan en el título.
La justificación y el objetivo del reporte se deben mencionar al final de la introducción.
La información del caso debe presentarse en forma cronológica, la molestia principal y datos relevantes de la historia clínica, incluyendo antecedentes de importancia, hallazgos del examen físico, la evaluación diagnóstica e intervenciones realizadas y resultados del seguimiento.
En la discusión indicar si lo mostrado en el reporte puede modificar la práctica clínica o sugerir una nueva hipótesis; si es posible mencionar conclusiones.
Imágenes en Medicina
Corresponden a imágenes ilustrativas, de propiedad del autor, no necesariamente infrecuente, que sean de utilidad para la educación continua. Deben ser acompañadas de una leyenda de extensión máxima de 150 palabras (en castellano e ingles), y un título de 10 palabras como máximo; el nombre y apellido de los autores (máximo dos), grados académicos o títulos y la afiliación institucional de los autores se presentan en hoja aparte como página del título. Las imágenes deben ser presentadas en formato JPG, GIF o TIF con una resolución de impresión mínima de 300 dpi, de lo contrario se debe adjuntar las fotos originales.
En caso no sea de propiedad de los autores, deben acompañar el permiso correspondiente para su uso y publicación.
Revisión de temas
Presenta el estado actual del conocimiento de un tema específico; o enfermedad; puede ser de dos tipos:
- solicitada directamente por el Consejo Editorial a personas expertas en el tema,
- presentada por profesionales interesados en un tema en particular. En este caso, el artículo será revisado por especialistas en el tema. Se publican en la sección Contribución especial.
No tienen estructura específica, pero en general deben tener las siguientes partes: Página del título, resumen (en el idioma original y en inglés), palabras clave, introducción, contenido, discusión y conclusiones, referencias bibliográficas y tablas, gráficos y figuras.
La extensión total del trabajo, excluyendo el resumen, agradecimientos, tablas, leyendas de figuras y referencias, no debe exceder las 5000 palabras. Se acepta como máximo de diez tablas, gráficos o figuras; el número máximo de referencias bibliográficas es 80.
El resumen es de tipo no estructurado (en el idioma original y en inglés), no debe exceder las 250 palabras. Al final agregar 3 a 6 palabras clave, que no se incluyan en el título.
Reseña histórica
Es un manuscrito sobre algún personaje o hecho ocurrido y su contribución al desarrollo de las ciencias biomédicas o de las políticas en salud.
No tiene estructura específica. La extensión total del texto del manuscrito, excluyendo el resumen, agradecimientos, tablas, leyendas de figuras y referencias, no debe exceder las 2 000 palabras. Puede incluir hasta seis tablas, gráficos o figuras; el número máximo de referencias bibliográficas es 20.
El resumen es de tipo no estructurado (en el idioma original y en inglés), no debe exceder las 150 palabras. Al final agregar 3 a 6 palabras clave, que no se incluyan en el título.
Cartas al editor
Documento que una persona, investigador o lector envía al editor de la revista para expresar sus opiniones, comentarios, observaciones o agregar información sobre un artículo o tema publicado en la revista o sobre un asunto de interés biomédico. Informes breves de una investigación o caso clínico también pueden ser realizados a través de carta al editor.
La extensión de la carta excluyendo las referencias no debe exceder las 600 palabras (Dos páginas escritas a doble espacio). Se puede incluir hasta dos tablas, gráficos o figuras y hasta 10 referencias bibliográficas.
La decisión sobre la publicación de las cartas recibidas queda a discreción del Consejo Editorial.
Aviso de derechos de autor/a
Los autores ceden sus derechos a la RMH para que esta divulgue el artículo a través de los medios que disponga. Los autores mantienen el derecho a compartir, copiar, distribuir, ejecutar y comunicar públicamente su artículo, o parte de él, mencionando la publicación original en la revista.
Declaración de privacidad
Política de Privacidad y Protección de Datos Personales
Los nombres y las direcciones de correo electrónico introducidos en esta revista se usarán exclusivamente para los fines establecidos en la Política de Privacidad y Protección de Datos Personales de la Universidad Peruana Cayetano Heredia.